
On August 11, 2021,Food and Drug Administration (FDA) approved SinoT’s ANDA(#213612)application for Paroxetine hydrochloride extended-release tablets(12.5mg,25mg).
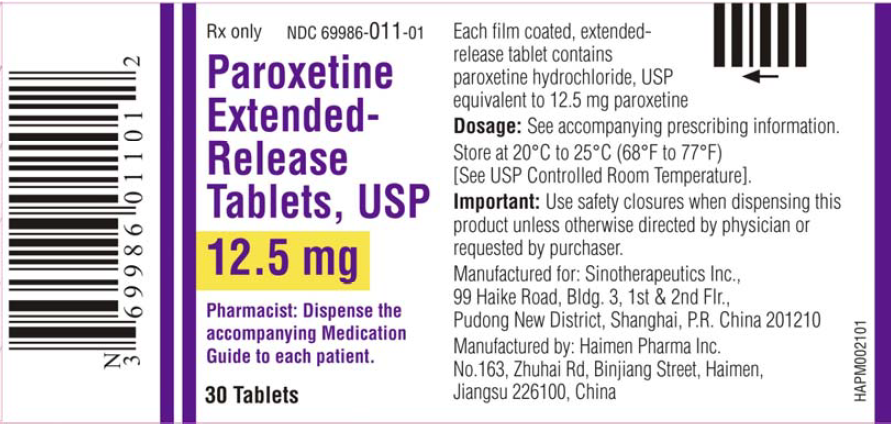

Paroxetine extended-release tablets are a selective serotonin reuptake inhibitor (SSRI), indicated in adults for the treatment of Major Depressive Disorder (MDD),Panic Disorder (PD),Social Anxiety Disorder (SAD) and Premenstrual Dysphoric Disorder (PMDD).
SinoT is a specialty pharma with focus on the research & development, commercialization and sale of complex generics and 505(b2) products. We dedicated to developing high quality medicines that meet with international standards. We’ll do more than our best to benefit the patients in the future.
Shanghai Head Office

99 Haike Road, Bldg. 3, 1st Flr., Pudong District Shanghai 201210, P.R. China
Manufacturing Site in Jiangsu

No. 163 Zhuhai Road, Haimen Area, Nantong City, Jiangsu Province
