
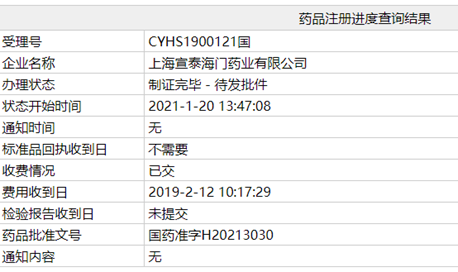
Recently, China's National Medical Products Administration (NMPA) approved SinoT's ANDA application for Posaconazole delayed-release tablets (100mg), this product has already been approved by FDA on August 2019 as the first generic drug.
POSACONAZOLE is a member of the group of triazole antifungals,which is used to prevent certain kinds of fungal or yeast infections. With the increasing number of high-risk patients such as cancer chemotherapy, transplantation, HIV/AIDS infection, and diabetes, the overall incidence of invasive fungal infections (IFIs) continues to rise. Different from other drugs used to treat IFIs, posaconazole can be used to prevent IFIs , so it has a broad market prospect.
SinoT is a technology-driven enterprise who is specialized in the research & development, formulation innovation, manufacture, commercialization and distribution of complexed generic products and novel formulation products (505B2). The company commits to the production and supply of pharmaceutical products that meet international quality standards, so as to bring good benefits to patients both at home and abroad, and to make its contributions to the well beings of the pharmaceutical industry, both in China and the rest of the world.
Shanghai Head Office

99 Haike Road, Bldg. 3, 1st Flr., Pudong District Shanghai 201210, P.R. China
Manufacturing Site in Jiangsu

No. 163 Zhuhai Road, Haimen Area, Nantong City, Jiangsu Province
